The U.S. Food and Drug Administration approved a clinical trial Friday that will allow Johns Hopkins University researchers to test a therapy for COVID-19 that uses plasma from recovering patients.
Arturo Casadevall, a Johns Hopkins infectious disease expert, proposed the use of convalescent plasma to treat critically ill COVID-19 patients and to boost the immune systems of health care providers and first responders. He assembled a team of physicians and scientists from around the United States to establish a network of hospitals and blood banks that can collect, isolate, and process blood plasma from COVID-19 survivors.
"The ability to carry out a prophylaxis trial will tell us whether plasma is effective in protecting our health care workers and first responders from COVID-19," said Casadevall, a Bloomberg Distinguished Professor who holds joint appointments in the Johns Hopkins Bloomberg School of Public Health and the Johns Hopkins School of Medicine.
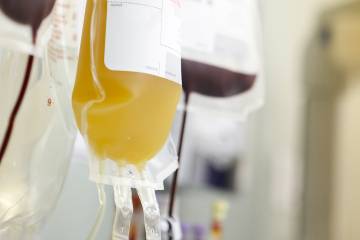
The strategy of isolating plasma is a long-established technology and recent advances make it as safe as a blood transfusion. The FDA's approval allows the team of researchers to begin testing the technique's effectiveness in boosting the immune systems of health care providers, first responders, and others at high risk of exposure to SARS-CoV-2, the virus that causes COVID-19.
Currently there are no proven drug therapies or effective vaccines for treating the disease. Casadevall and his team believe using plasma from recovered patients could provide immediate immunity to the most at-risk individuals.
"Plasma transfusions are critically important and used every day to save lives of patients who are bleeding," said research team member Aaron Tobian, a professor of pathology, medicine and epidemiology. "It is incredibly important for us to determine whether convalescent plasma will also be able to save the lives of individuals infected with COVID-19 and prevent infection of COVID-19."
Last week, Casadevall's team received $4 million to fund the research from Bloomberg Philanthropies and the State of Maryland.
"Dr. Casadevall and his colleagues from across Johns Hopkins and partners around the nation are working with creativity and persistence to face this disease head on," said Johns Hopkins President Ronald J. Daniels. "Arturo's and his partners' work reflects Johns Hopkins' abiding commitment to collaboration and discovery that serves humanity. We are grateful for the FDA's swift support for this life-saving work and the promise it holds for so many, particularly our frontline healthcare workers, in this extraordinary time."
At Hopkins, the efforts to deploy convalescent plasma against COVID-19 is led by an interdisciplinary team that includes Evan Bloch and Shmuel Shoham of the School of Medicine and Andy Pekosz and David Sullivan from the Bloomberg School of Public Health.
"We are grateful and encouraged that researchers can move forward with this important research aimed at protecting those most at risk, our front-line health care providers and first responders," said Bloomberg School Dean Ellen J. MacKenzie. "We also applaud the researchers' vision in the early stages of this pandemic to explore using blood plasma to treat critically ill patients."
Update: The Red Cross is helping coordinate blood donations for recovered COVID-19 patients..
Posted in Health, University News
Tagged arturo casadevall, coronavirus, covid-19