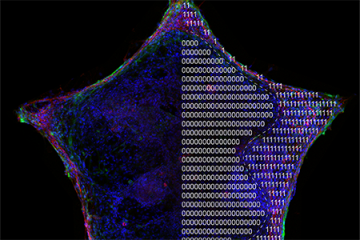
Researchers and clinicians don't fully understand why some cancers spread and others do not. What they do know is that when cancer does spread, it dramatically decreases survival rates.

Image caption: Konstantinos Konstantopoulos
If physicians could predict the likelihood that primary tumors will metastasize, they would be able to choose the best treatment options for patients. However, current testing only reviews tumor genetics, which can mutate and change.
Chris Yankaskas, a PhD candidate in the Department of Chemical and Biomolecular Engineering at Johns Hopkins University, wondered if he could predict metastasis from a different angle, by instead looking at the cancer cell's phenotype, or observable cell characteristics and behaviors. Under the direction of Konstantinos Konstantopoulos, a professor and core faculty member of the Institute for NanoBioTechnology, Yankaskas and a team of researchers created the Microfluidic Assay for Quantification of Cell Invasion, or MAqCI, a diagnostic tool and method for predicting breast cancer metastasis by looking at two key cell behaviors needed for metastasis instead of tumor genetics.
"The complexity of cancer progression and differences between each patient's cancer cells make metastasis hard to predict on a case-by-case basis," said Yankaskas. "We aim to continue working in breast cancer using cells from patients' biopsies and hope to expand the technology to other cancer types."
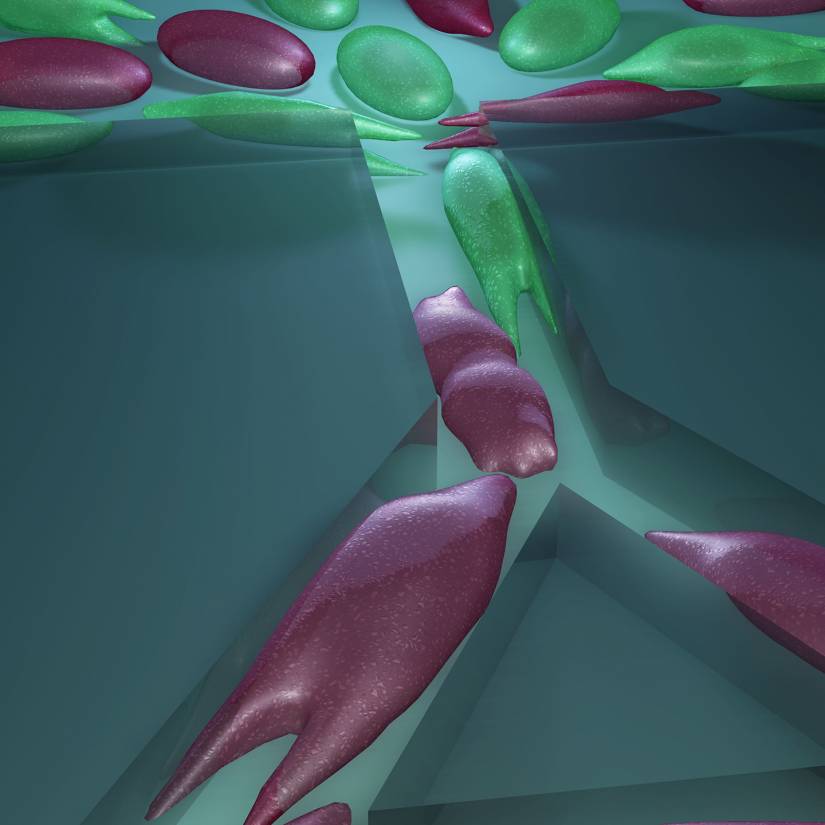
Image caption: Breast cancer tumors contain a mixture of cells with different abilities, and the proportion of aggressive cells within a tumor determines its likelihood to metastasize. MAqCI identifies aggressive breast cancer cells (in purple) based on their ability to move from a feeder channel into narrower channels.
Cancer treatments are strenuous on the body and can be costly. Some patients need chemotherapy, radiation, surgery, targeted therapies, or a combination of all of the above. MAqCI can help clinicians and patients identify the most appropriate treatment for aggressive cancers and avoid over-treating less aggressive cancers.
To develop their device, Yankaskas first had to train MAqCI (pronounced mak-see) to recognize the characteristic behaviors of normal breast epithelial cells (their control group), non-aggressive breast cancer cells, and aggressive/metastatic breast cancer cells. Once those parameters were established, the team then used independent cell populations, including breast cancer patient-derived specimens, to validate that MAqCI could correctly measure and characterize the cells.
The test measures two key cell behaviors that are required for metastasis to occur: cell motility, the measure of how capable cells are of traveling to distant sites within the body, and proliferation, which is how much they are multiplying.
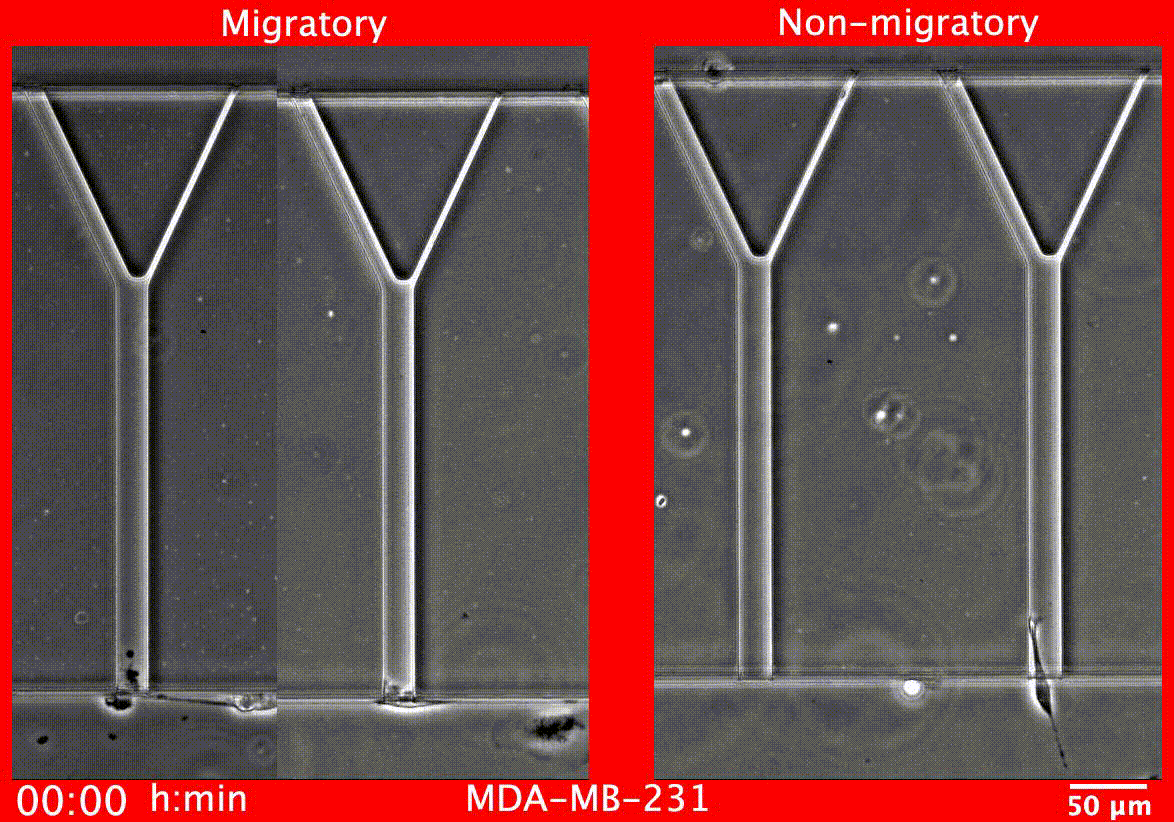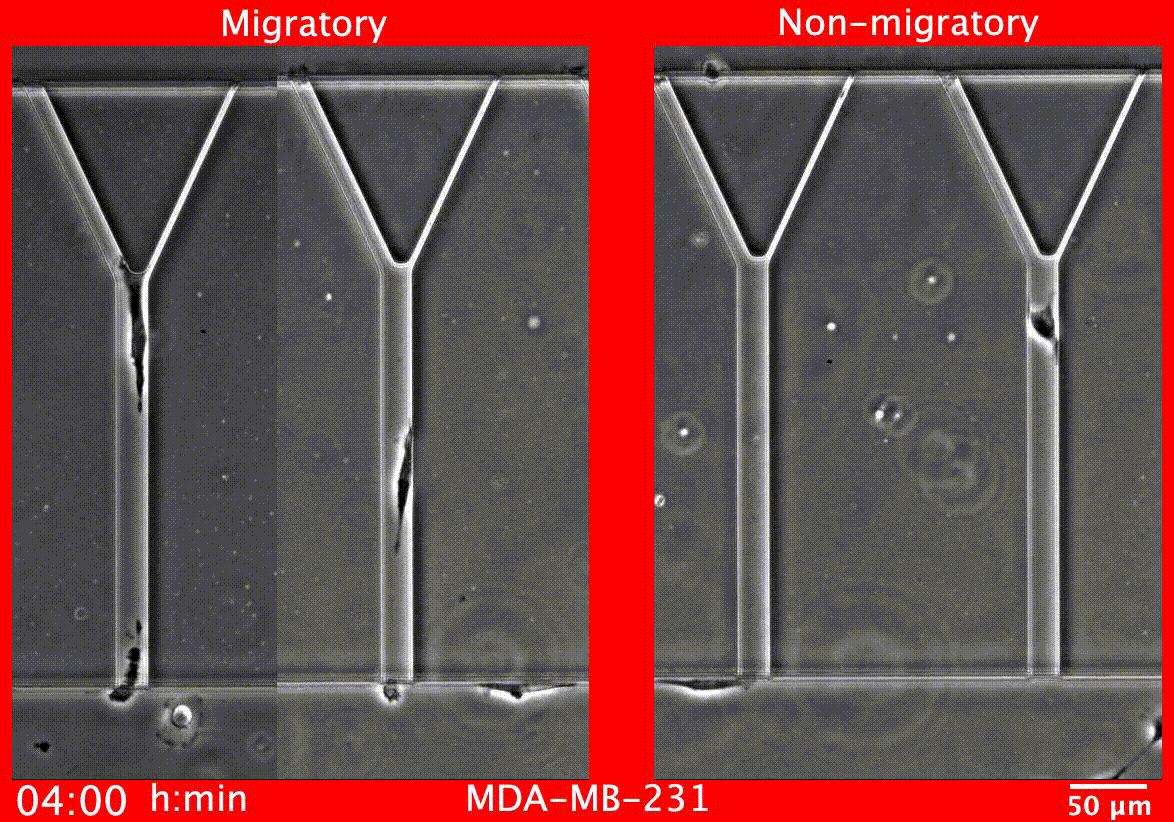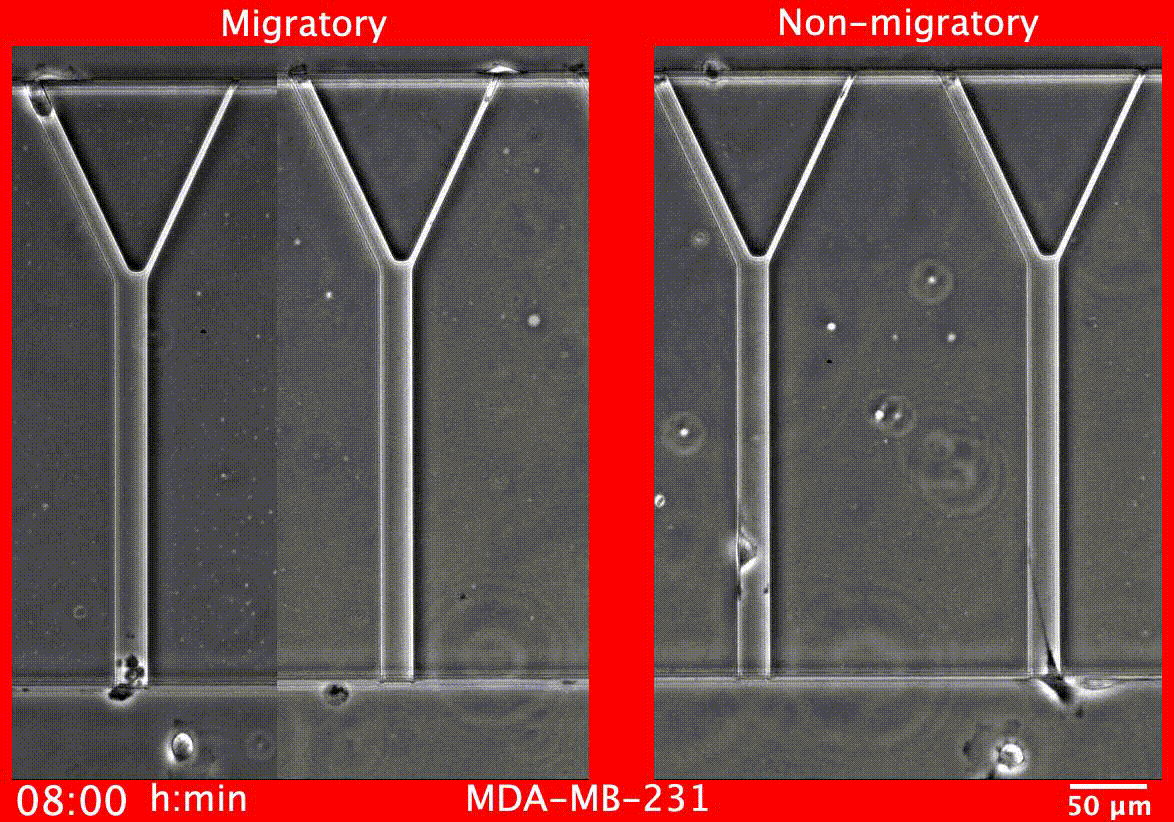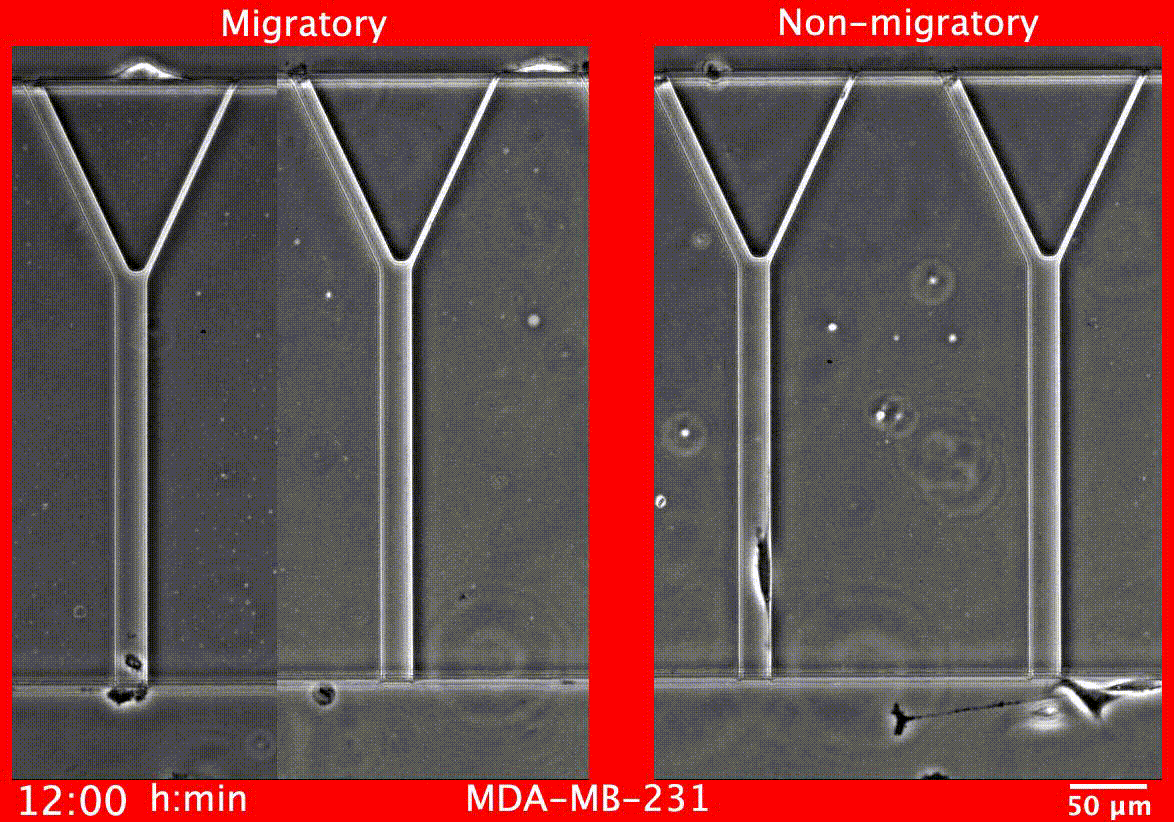
Image caption: Migratory breast cancer cells (left) deform to fit into narrow tubes
Results, published in Nature Biomedical Engineering, show that MAqCI is accurate, sensitive, and specific enough to predict if a breast cancer population will metastasize. The technology has potential clinical use because it uses small sample sizes, delivers results within one to two days, and can isolate these cells for further characterization.
Another advantage of MAqCI testing is that it looks at observable characteristics of cells and is relatively simple and easy to interpret, unlike genetic screening. Predicting if a cancer population is able to metastasize can be difficult, and this behavioral approach offers a simpler, more effective way of making a prediction.
"MAqCI has the potential to diagnose a tumor's metastatic propensity and screen therapeutics that target metastasis-initiating cells on a patient-specific basis for personalized medicine," Konstantopoulos said. "We are currently testing our assay to predict survival expectancy of brain cancer patients. We believe that MAqCI will be a great tool for diagnosis, prognosis, and precision care of patients with solid tumors."
Posted in Health
Tagged breast cancer, chemical engineering, biomolecular engineering