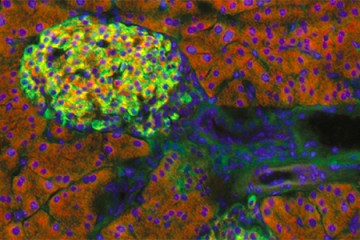
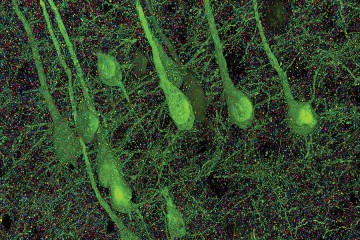
Humans and other vertebrates depend on a portion of the brain called the hippocampus for learning, memory, and their sense of location. Nerve cell structures in the adult hippocampus are sustained by factors whose identities have remained largely mysterious so far.
Now, research led by a Johns Hopkins University biologist sheds light on the subject, potentially pointing the way to a better understanding of how the structure of nerve cells in the adult hippocampus may deteriorate, which can lead to Alzheimer's disease and other neurological disorders.
"It's literally a black box," said Rejji Kuruvilla, an associate professor in the Department of Biology who led the research team, referring to the biochemical signaling systems that support the neurons in the adult brain. "To get a handle on repairing the adult brain you first need to know what those signals are."
In a paper that appears in the Proceedings of the National Academy of Sciences, Kuruvilla and eight other scientists from two research institutions report on results showing that a particular protein that has primarily been studied for its role in early animal development plays a surprising role in maintaining the structure of hippocampal neurons in adult mice. Chih-Ming Chen, a graduate student from Kuruvilla's laboratory, was the lead author on the study.
Also taking part in the work were scientists from the Scripps Research Institute Florida, and the Department of Neuroscience at Johns Hopkins University School of Medicine.
The team studied a protein called Wnt5a, which belongs to a family of proteins that have been primarily studied for their functions during embryonic development and in nurturing neurons as the young brain forms. Using mice genetically altered to remove Wnt5a from the hippocampus, the team showed that the protein's absence did not affect hippocampus development in young mice, but instead resulted in striking degradation of specific nerve cell structures called dendrites, which resemble clusters of tree branches, in adult mice. These findings suggest that the protein plays an important role in maintaining dendrite structures as the mouse ages.
The team went further by showing that when the Wnt5a protein was reintroduced after the dendrites had started to deteriorate in aged mice, the nerve cell structures were restored—to a degree the scientists did not expect.
"I was surprised at the scale of growth we saw in the adult brain," Kuruvilla said. The nervous system is thought to be dormant in growth in adults, she said.
The team also tested the ability of mice lacking Wnt5a in the hippocampus to perform learning and memory tasks. Behavior tests were run in a Morris Water Maze designed to show how well mice use spatial cues to navigate in a water pool, and how long it takes them to learn the location of a hidden platform that they can then use to escape from the water. They found that mutant mice—those without Wnt5a—were poor learners and had attenuated memory and their performance in behavioral tasks became progressively worse with age.
"Together, the findings from the Morris Water Maze test support an essential role for Wnt5a in the acquisition of spatial learning and memory storage in adult animals," the authors wrote.
In the brain, the hippocampus is the seat of short and long-term memory and also governs spatial orientation. Studies in humans have shown that the brains of people suffering with Alzheimer's disease have structural alterations in dendrites, with changes particularly striking in the hippocampus.
Kuruvilla said the experiments suggest avenues for further research on what may cause shrinkage of dendrites during neurological disorders. However, she emphasized that it is premature to extrapolate these results in mice to humans.
While Kuruvilla was careful not to overstate the significance of the findings or how they may apply to cognitive disorders in humans, she said the results at least bring attention to the significance of studying molecular signals that maintain neurons in the adult brain. Compared to the wealth of information on signals that help in formation of neuronal connections in the developing brain, we know far less of how brain structure is sustained in adult life, she said.
Posted in Health, Science+Technology
Tagged biology, neuroscience, neurology, memory