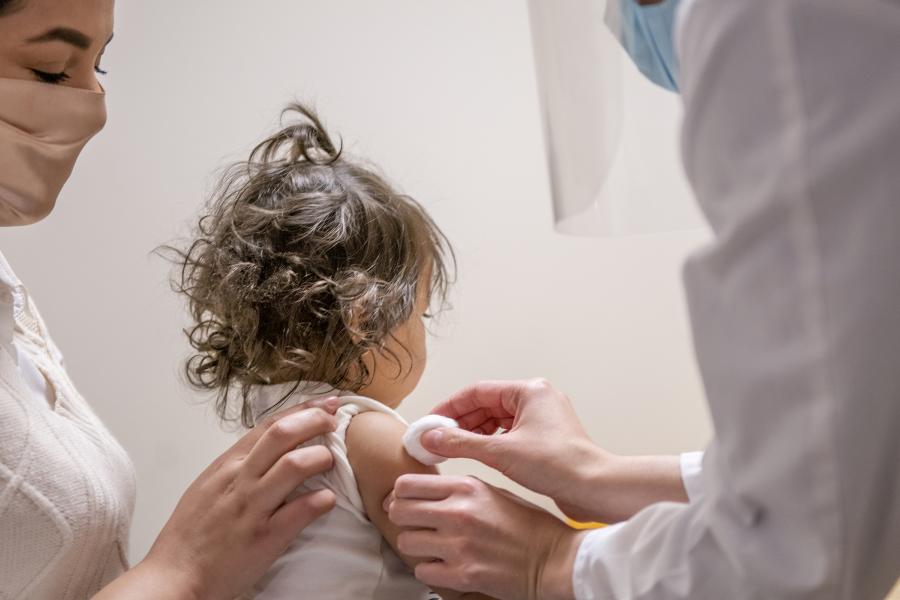
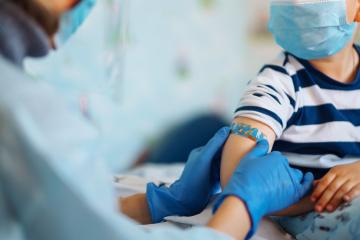
U.S. children older than 6 months are now eligible to receive COVID-19 vaccinations after the U.S. Food and Drug Administration and Centers for Disease Control and Prevention recently authorized vaccines from Moderna and Pfizer/BioNTech.
The federal agencies last week granted emergency use authorization for Pfizer/BioNTech's vaccine for children younger than 5 years old and for Moderna's vaccine for children younger than 6 years old.
"Both vaccines appeared to be safe, with similar side effects as in older children and adults, such as fever and soreness at the site of injection," said William Moss, vaccinology lead for the Johns Hopkins Coronavirus Resource Center. "However, it is critical that safety monitoring continue after authorization to detect any rare adverse events."
'Both vaccines appeared to be safe with similar side effects as in older children and adults.'
Moss, a pediatrician and executive director of the International Vaccine Access Center, said he does not expect vaccination coverage in the United States to greatly improve due to the authorizations, which apply to the nearly 20 million U.S. children younger than 6 years old.
"I suspect demand for COVID-19 vaccines in children younger than 5 years will be modest at best," Moss said. "There are some parents who have been waiting a long time to have their pre-school children vaccinated, but only 36% of children 5 to 11 years of age have received one dose and only 29% are fully vaccinated. Vaccine coverage levels in children younger than 5 years will be even lower, I suspect."
The percentage of the U.S. population that is considered fully vaccinated has stubbornly remained at around two-thirds, according to Coronavirus Resource Center data. For the population over 5 years old who previously have been eligible only for the Pfizer/BioNTech vaccine, the percentage increases to 71%. About 91% of those over 65 years old are fully vaccinated, according to the CDC.
A CDC survey indicated that about a third of U.S. parents plan to get eligible children vaccinated.
The Moderna COVID-19 vaccine for children 6 months old through 5 years is a two-dose regimen, Moss said. The Pfizer/BioNTech COVID-19 vaccine for children 6 months old through 4 years is a three-dose regimen, "as their two-dose regimen did not lead to sufficiently high levels of protective antibodies," he added.
"The studies of these vaccines were relatively small compared to the original trials in adults but FDA experts found the Moderna vaccine to be safe and effective in young children," Moss said. "This was based in part on levels of neutralizing antibodies following vaccination. However, effectiveness in preventing symptomatic infection was only 51% in children 6 months to 2 years of age and 37% in children 2 to 5 years of age, certainly lower than what we would hope for. Children in this age group will likely need a third dose for better protection, as is the case for older children and adults."
'The benefits of the vaccines still far outweigh the risks.'
The FDA stated that the three-dose regimen of Pfizer/BioNTech COVID-19 vaccine appeared to be effective in preventing symptomatic COVID-19 in young children, Moss said. "But only 10 of the 1,415 children in the study became ill—two among the vaccinated children and eight who received the placebo," he added. "This is too few cases to draw definitive conclusions but certainly suggestive. The three-dose series also resulted in protective levels of antibodies, providing further evidence of benefit."
Brian Garibaldi, the CRC clinical lead, said vaccines will be beneficial to children and the broader community.
"While the risk of severe disease in children is low, the benefits of the vaccine still far outweigh the risks, both in terms of individual protection but also in terms of helping to slow down community transmission," Garibaldi said. "Children can also get long COVID and vaccines are likely to reduce the incidence of long COVID—by preventing infection in the first place — and may reduce its severity in those who do develop long term symptoms, although the data on this is less clear."
While Pfizer's COVID-19 vaccine for children ages 5 to 11 was authorized last November, it has taken longer than anticipated to expand eligibility to younger children for a number of reasons, according to Ruth Karron, director of the Johns Hopkins Center for Immunization Research, who discussed the delay during an interview with the Bloomberg School of Public Health's podcast, Public Health On Call.
"When we started to make COVID-19 vaccines in 2020, I don't think we appreciated the full burden of COVID on young children," she said. "Certainly we appreciated the indirect effects on schooling and other social activities. But it took us a while to fully appreciate the direct medical effects."
Karron highlighted the novel aspect of the mRNA platform used to develop the vaccines.
"It's highly effective, but it is also inflammatory. Anyone who's gotten these vaccines knows that you can have low-grade fever, chills, and aches of various kinds," she said. "And one of the things we know about children is that their inflammatory reactions are heightened. Children get fevers, for example, much more frequently than adults do. The companies and the [government] bodies that regulate these vaccines wanted to make sure that they were being evaluated as carefully as possible, to ensure that a vaccine would be safe to administer to children."
Posted in Health
Tagged vaccines, coronavirus, covid-19 vaccine, coronavirus resource center