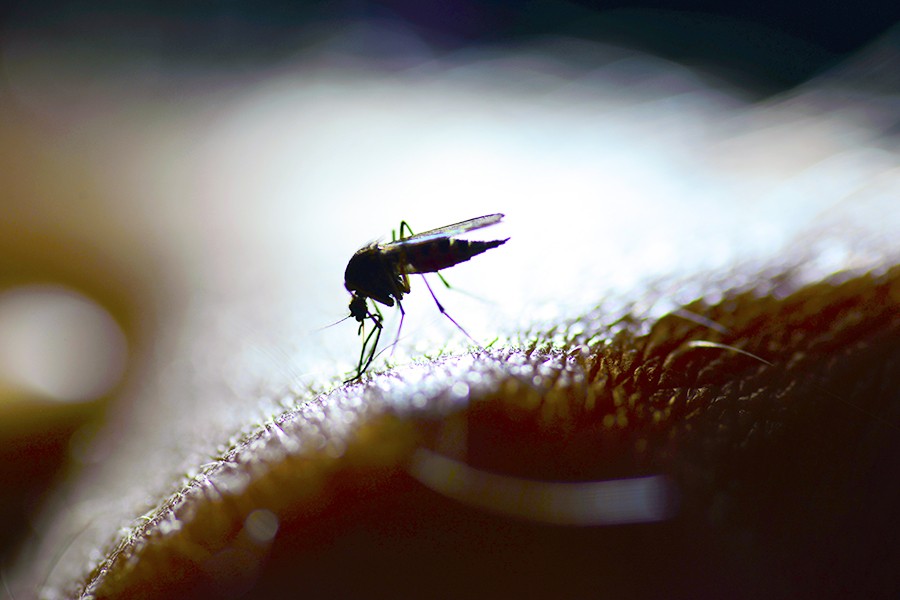
Two new papers by researchers at the Malaria Research Institute at the Johns Hopkins Bloomberg School of Public Health report successes for highly promising strategies against malaria, a disease that kills more than 400,000 people each year, mostly children age 5 and under in sub-Saharan Africa.
Malaria is spread by female Anopheles mosquitoes carrying the malaria parasite. One promising way to prevent malaria, in addition to traditional approaches such as bed nets and insecticide, is to modify the mosquitoes so they are no longer capable of spreading the parasite to humans.
The studies discovered different ways by which resistance to the malaria parasite can spread into a mosquito population, potentially paving the way for the development of self-propagating malaria control strategies.
One team of researchers discovered a strain of bacteria that can spread rapidly and persist long-term among malaria-carrying mosquitoes. A genetically modified version of that strain strongly suppresses development of the malaria parasite, making the mosquitoes much less likely to transmit these parasites to humans.
A second research team discovered that a genetic modification that boosted the immune system of malaria-carrying mosquitoes not only suppresses malaria parasites in the insects but also can spread quickly in a test population by changing the mosquitoes' mating preferences.
These findings, which will be described in papers that will appear in Friday's issue of Science, could lead to developing bacteria and mosquitoes that would be released into mosquito populations in the wild, then propagate on their own to reduce malaria transmission to humans in endemic areas. These strategies are designed to be complementary and would be used in conjunction with things like bed nets and insecticides to diminish the transmission of disease.
Study 1: Fast-spread bacteria
The discovery of the new mosquito-infecting bacterial strain was a chance event.
"We were working with a different bacterium when a researcher on the project happened to find evidence of a bacterial colony in our mosquitoes' ovaries," says senior author Marcelo Jacobs-Lorena, a professor in the Bloomberg School's Department of Molecular Microbiology and Immunology and a member of its Malaria Research Institute. "That was unusual—normally we find bacteria only in the mosquito gut."
His team soon characterized these odd microbes as a strain of Serratia bacteria, and dubbed them Serratia AS1.
Also see
Jacobs-Lorena and other researchers have been developing genetically engineered bacteria that can infect mosquito populations and kill the malaria parasites the mosquitoes harbor without harming the mosquitoes themselves. Getting such bacteria to spread efficiently has been a key challenge, but experiments revealed Serratia AS1 to be almost perfect for the task. Jacobs-Lorena and colleagues found that, unlike other mosquito-infecting bacteria, Serratia AS1 are easily transmitted from males to females during mating and from female mosquitoes to their offspring. The bacteria also colonize the mosquito gut, where malaria parasites develop.
In one experiment, the scientists used Serratia AS1-laden sugar bait to infect males and virgin females representing just 5 percent of a mosquito test population. They found that in the next generation, the bacterial strain was present in 100 percent of the larvae and adult mosquitoes. The researchers followed this mosquito population for two more generations—about a month's time—and found that the bacteria remained ubiquitous. The results suggest that Serratia AS1 bacteria are likely to spread and persist long-term in wild mosquito populations.
The scientists modified Serratia AS1 by adding genes for five potent antimalarial proteins that were developed in the laboratory. Powered by these antimalarial proteins, the bacteria strongly inhibited malaria development in colonized mosquitoes, reducing the levels of an early stage form of the parasite by more than 90 percent compared to mosquitoes that didn't contain the modified bacteria.
Further experiments showed that the modified Serratia AS1 bacteria don't have a significant effect on mosquito lifespan or fertility.
"So far all indications are that these anti-malarial proteins are universally effective against malaria parasites, and the Serratia AS1 bacteria that carry them can go into any malaria-carrying mosquito species," Jacobs-Lorena says.
He and his colleagues now plan to follow their small-scale laboratory experiments with larger-scale experiments at the Malaria Research Institute's "Mosquito House" research facility in Macha, Zambia. After that, the scientists hope to be able to release Serratia AS1 bacteria in an isolated island-like environment to study how the microbes spread among wild mosquitoes.
Study 2: Boosting the immune system
In the second study, a team led by George Dimopoulos, professor in the Bloomberg School's Department of Molecular Microbiology and Immunology, made small modifications to the DNA of malaria-transmitting Anopheles mosquitoes to boost the activity of immune genes in the insects. The enhanced immunity made the mosquitoes more resistant to infection by malaria parasites, and thus less likely to transmit the parasites to humans.
That result was expected. What wasn't expected was the unusually high efficiency with which the modified mosquitoes spread their genetic modification to ensuing generations in a mixed population of modified and unmodified, wild-type mosquitoes.
Investigating this surprising result, Dimopoulos and colleagues found that boosting the mosquitoes' immune genes also boosted their defenses against bacteria, reducing the normal bacterial load and altering the normal mix of bacterial species in the mosquito intestine and reproductive organs. This change in the insect "microbiota" in turn led to a change in mating preferences, such that modified male mosquitoes began to prefer unmodified, wild-type females, while wild-type males began to prefer modified females.
"We believe that by changing the microbiota we're changing the scent of modified mosquitoes—which in turn alters mating preference," Dimopoulos says. "It's the perfect change in mating preference in this case, because it maximizes the chances of producing genetically modified offspring when mosquitoes compete for mates."
It's important to note that the DNA modifications only involved an alteration of the mosquito's own gene activity, and not the introduction of foreign genes. Other laboratories are developing a different method to release mosquito genetic modifications into test populations using complex "gene drive" DNA modifications. These essentially override the normal dynamics of inheritance to force new genes into nearly 100 percent of the offspring of mating mosquitoes. While potentially very promising, gene drives are still controversial because of their artificiality, complexity, and potential for long-term instability.
The findings from Dimopoulos and his colleagues show that even a subtle genetic modification that merely boosts the activity of existing genes can spread quickly into a mosquito population—without the need for a complex gene drive system. The two systems could also be combined, however, to maximize the spread of malaria-resistant mosquitoes.
Dimopoulos's modified mosquito population has now been living in a colony in his laboratory for more than seven yearsand has retained its high level of resistance to malaria for all that time, without any apparent adverse side effects from the genetic modification.
"These mosquitoes haven't changed in terms of feeding behavior or any other things that could be of concern," Dimopoulos says.
Dimopoulos and his colleagues now plan to study the effects of their genetic modifications in larger settings, such as the institute's Mosquito House in Zambia.
Both studies were supported by the National Institute of Allergy and Infectious Diseases and the Bloomberg Philanthropies. The first study was also supported by the Strategic Priority Research Program of Chinese Academy of Sciences and the National Nature Science Foundation of China.
Tagged epidemiology, malaria