Heart attacks scar the heart, leaving patients vulnerable to heart rhythm disorders that can lead to sudden death. While not all who have experienced a cardiac infarction will develop an arrhythmia, if they do, it will typically happen about three years post-attack. In these patients, fat penetrates the heart wall in the region of the scar after three years, as well. Until now, however, the relationship between those fat deposits and the development of arrhythmias was unclear.
A combined clinical and computational study led by researchers at Johns Hopkins and the University of Pennsylvania reveals that fat intermingling with scar tissue is the driving force behind the development of heart rhythm disturbances. The team's results were published October 6 in Nature Cardiovascular Research.
The Hub sat down with the study's corresponding author, Natalia Trayanova, the Murray B. Sachs Professor of Biomedical Engineering at Johns Hopkins, to talk about these new results. Co-director of the Alliance for Cardiovascular Diagnostic and Treatment Innovation, and a member of the Institute for Computational Medicine, Trayanova is a pioneer in the use of 3D virtual replicas of the heart and its electrical functions, which are personalized to patients with various heart conditions. She partnered on this study with Jonathan Chrispin, assistant professor of medicine and director of ventricular arrhythmia research at the Johns Hopkins School of Medicine, and Saman Nazarian, professor of medicine at the Hospital of the University of Pennsylvania.
Can you describe how you conducted this study?
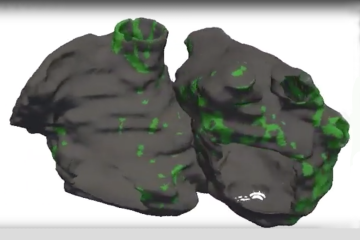
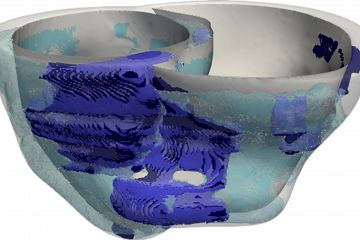
We needed to understand whether scarring in the heart or the presence of fat in the heart wall—or both—contribute to arrhythmias in post-infarction patients. To do so, we needed to first analyze images of patients' hearts and see where scarring and fat penetration occur. However, since scarring in the heart is visualized on contrast-enhanced MRI, while fat can only be seen on contrast-enhanced CT scans, there were no patient records that had both types of imaging data already available. So we decided to conduct a prospective clinical study, where post-infarction patients who were scheduled to undergo an invasive treatment for their arrhythmias had both an MRI and a CT scan taken. Using funds from an NIH grant between researchers at the University of Pennsylvania and those of us at Johns Hopkins, 24 patients were enrolled at both institutions. My lab used the patients' MRI and CT images to create the first-of-their-kind CT- and MRI-based digital models of the patients' hearts, and used them to understand how heart rhythm disorders arise in these patients. The results from the simulations, together with the electrical recordings acquired from the hearts of the patients during the treatment procedure, helped us figure out the role of the penetrating fat in lethal arrhythmias. This was a true combined clinical and computational study, with patients prospectively enrolled at two different clinical centers.
What did you find out?
Using the personalized heart models executed by Eric Sung, the paper's first author and a student in my lab, we determined that larger amounts of fat in a patient's heart wall was predictive of an increased likelihood of arrhythmias; in contrast, a larger amount of scarring was not associated with an increased arrhythmia likelihood. Using both the patient heart models and the clinical recordings acquired during the procedure, the presence of fat in the heart wall was found to slow down conduction of electrical signals that underlie heart rhythm, making them more likely to behave in a disorderly fashion. Overall, the study demonstrated that penetrating fat rather than scarring in the heart is the primary reason for arrhythmia occurrence in these patients.
What are the real-life implications of what you learned?
Our findings implicate the fat that penetrates the walls of the heart as a new, significant player in post-infarct arrhythmias. This finding challenges the conventional wisdom, which considers scarring in the heart as the main reason for heart rhythm disorders in patients with infarction. We envision that this new knowledge will motivate novel, patient-specific therapeutic strategies to mitigate heart rhythm disorders. For instance, knowledge of how fat is distributed in the heart will affect how catheter ablations are delivered to treat arrhythmias. We also envision implementation of new pharmacological strategies to decrease the extent of penetrating fat in post-infarct patients, thus decreasing the occurrence of arrhythmic events.
What are your next steps with this research?
We will continue enrolling patients to increase the size of the patient cohort and ensure that our findings remain valid. We also intend to examine whether penetrating fat plays a role in heart rhythm disturbances in other heart diseases.
Posted in Health, Science+Technology
Tagged natalia trayanova, cardiac health, arrhythmia