How do you take your coffee? With cream? Sugar? How about with a heaping helping of chemistry?
Noah Yan, the instructor behind the Intersession course Roasted: The Molecular Gastronomy of Coffee, hopes his students will think of the person who prepares their morning brew as more of a chemist than a barista as Yan delves into the art and science behind what makes the perfect cup of coffee.
The course details the chemical and biological reactions that create the flavors in a cup of coffee, analyzing the roasting process, preparation methods, and distinctions between sweeteners and creamers, all while looking into the health benefits and effects of each.
"Coffee is this interesting intersection between biology and chemistry," Yan says. "On one end, it's how the beans grow, and on the other it's in how you brew it and how you roast it. There are these reactions and processes that we can see unified when we make coffee."
In each class session, students compare the tastes of different coffees while Yan explains their distinctions through chemistry formulas illustrated as lovable cartoons.

Image caption: Noah Yan teaches Roasted: The Molecular Gastronomy of Coffee Intersession course
Image credit: Will Kirk / Johns Hopkins University
Yan graduated from Johns Hopkins University's Krieger School of Arts and Sciences with a degree in molecular and cellular biology this past December. He said the idea for the class has been percolating in his mind since his freshman year.
"There's a coffee shop where I'm from in the Bay Area called Philz, and what took me aback was how much more I enjoyed Philz's coffee over just about every other place's. I had to figure out what they were doing differently," Yan said.
Turns out, it all starts with the bean.
Chemically, a coffee bean is made up of chlorogenic acids, carbohydrates, caffeine, proteins, amino acids, lipids, and water. Decisions made while growing, collecting, and roasting the beans affect these elements and give the roast its final flavor.
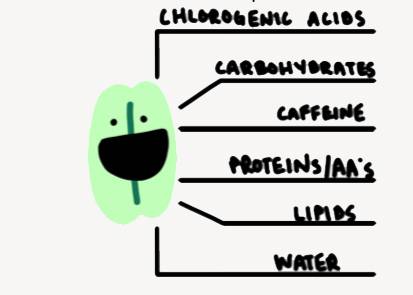
Image caption: Noah Yan's coffee bean illustration from the course
Coffee plants are divided into two varieties—arabicas and robustas. Each plant has its own advantages and flavors dependent on the geography and humidity where it is grown. Arabica plants grow at a higher elevation with lower moisture, while robusta grows less widely and is more resilient to pests. While both have their place in coffee culture—robustas are popular in espresso and instant coffee—most American bistros focus on the arabica for its wide and complex flavor.
After the fruits of the coffee plants are collected, they are stripped to the bean or seed. Choosing a method to extract the seed is an early influence in the final flavor of the coffee. Growers can use water to power wash the flesh of the fruit off, allow the sun to bake it off, or allow microbes to ferment the flesh until it falls off.
Each method affects the makeup in the seed in its own way by adding additional microbes, increasing water content, or heating the bean prior to roasting, for example.
The bean—still green at this point—must then be roasted to break down the simple and bitter flavors into the distinct taste of a cup of joe. As the beans are roasted, water evaporates, breaking the H2O links in sucrose—large sugars which have difficulty combining with other molecules—into glucose and fructose.
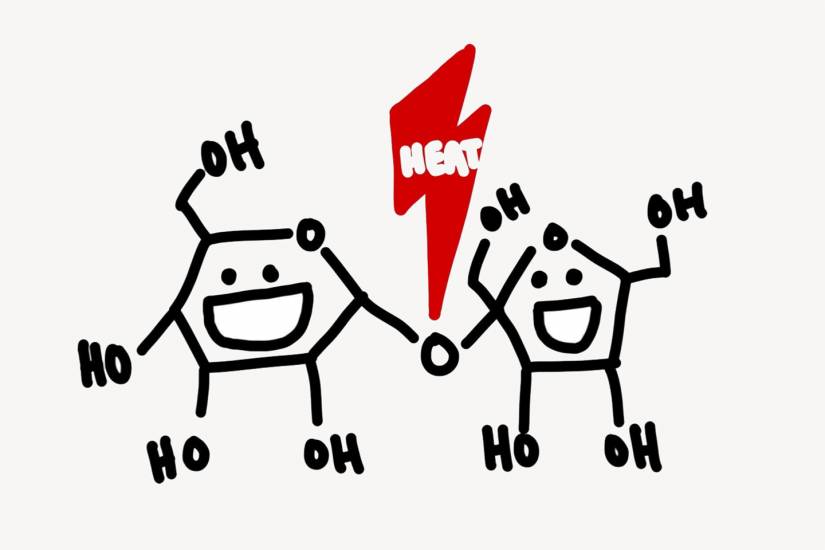
Image caption: When heat is applied, H2O evaporates, breaking the sucrose bonds and creating simpler sugars
Image credit: Illustration by Noah Yan
Some of these simpler sugars produce caramelans, which provide nutty, fruity, and buttery flavors, while others react with proteins in a browning Maillard reaction, creating a heterogeneous collection of sugars that create a more complex sweetness.
As the water evaporates from the bean, it forms a crack where steam can rush out. At 200 degrees Celsius, a light roast has been created that is fragrant, floral, and fruity.
If the beans continue to roast, chlorogenic acids that give coffee its fruity taste decompose into new bitter compounds, giving dark roasts their signature flavor. At these higher temperatures, sugars are also burned off as CO2 and H2O, creating a less-sweet brew.
After the beans are roasted to the desired level, how they're prepared for brewing will affect the final flavor.
First, the roasted beans are ground, affecting how easily water can interact with the bean and how flavors can be extracted from it, and the water temperature will affect how many compounds seep out of the bean. This final process of coffee brewing requires careful attention. If the water is too hot, it will break down the complex compounds formed in the roasting process, creating a less appealing flavor. If the grounds are kept in the water too long, more of the bitter compounds will be extracted, overpowering the taste of the brew.
At the end of the long process, the chemical reactions have completed, the flavors intertwined into something unique, and you finally have a cup of coffee to enjoy. Considering the long complex journey from the plant to your cup, next time you pick up your morning joe, maybe go leave an extra tip for your favorite chemists.
Posted in Student Life
Tagged chemistry, intersession









