The molecules that make the body of a person or any other organism function can be summed up, for the most part, in a word: proteins.
These molecules carry out almost all processes in living organisms, including moving other molecules from one place to another, replicating DNA, conveying genetic information from genes to cells, controlling immune response, driving metabolism, and building muscle. Not all protein molecules are created equal, though, and some are better understood than others.
Now, a team of scientists led by a Johns Hopkins University biologist has cracked a key part of the mystery surrounding a distinct type of protein that emerged less than 30 years ago. The finding, reported in the online journal eLife, could eventually lead to treatments for diseases that range from cancer to neurological disorders.
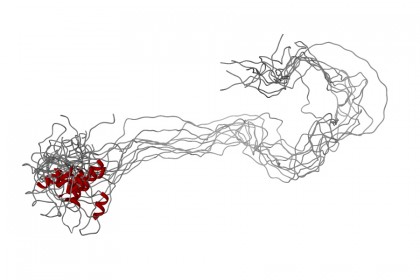
Image caption: An example of an intrinsically disordered protein in its extended, unfolded state
Image credit: LP Kozlowski
These proteins are called "intrinsically disordered proteins," which make up about 40 percent of all proteins. They constitute the majority of proteins involved in a process called "transcription"—how the instructions in genetic code are conveyed to cells and ultimately body tissues.
It's not clear exactly how errors in transcription affect human health, but it is known that these errors are involved in most cancers, said Vincent Hilser, professor and chair of the Johns Hopkins Department of Biology. He added that it's not possible to say when this new research will translate into improved treatments, but that understanding how these intrinsically disordered proteins work is a critical step toward that.
"It's probably going to be the case that to understand many, if not most, cancers, you're going to have to understand [disordered proteins]," he said.
Until the early 1990s, scientists only knew of "structured" proteins existing as unique shapes that respond when a regulator molecule binds to them, changing their shape and controlling their function. These protein molecules have been compared to origami creations for their ability to fold into a particular shape.
Anything showing up in experiments that did not fit that profile was often dismissed as some problem with the experiment, or an anomalous form that was not biologically significant.
These outliers have since been recognized as a legitimate form of protein, although given a somewhat disparaging name. They don't fold up, they don't assume any unique shape at all other than strands of spaghetti, as Hilser puts it. Hence the "disorder" in the name, as opposed to "structured" proteins—and part of the mystery.
If the structure is the mark of the regulating molecule doing its work—determining protein activity and function—then what to make of proteins that do not do that? What controls the activities of these shapeless strands?
The scientists, nine of whom are from Johns Hopkins and one from the University of Houston, set out to answer that question. They chose for their study a disordered protein taken from human cells called glucocorticoid receptor, which regulates genes that control, among other functions, metabolism and immune system response.
By manipulating segments of the protein in the lab, they were able to show how one portion acts on another, and that the disordered protein creates versions of itself to act almost in place of regulator molecules that govern its activity. The disordered protein uses an activation-repression dynamic—described by Hilser as similar to attracting and repelling magnets—between sections within the disordered chain to regulate its own activities and those of other proteins.
"Our work uncovered the language of how these spaghetti pieces communicate," Hilser said. "We showed that those pieces of spaghetti interact with each other sort of like attracting and repulsing magnets, creating a kind of tug-of-war, and that the body can make different versions of the protein to tune which part wins the tug-of-war."
Yet to be explained, he said, is how the interactions among these proteins and the sub-sections happen and how all this can ultimately be used to treat disorders that emerge when things go awry with these molecules central to almost all life function.
Posted in Health, Science+Technology
Tagged biology