Virginie McNamar often watches her nearly 3-year-old son, Tyler, run around the darkened basement playroom. She isn't sure why he prefers the lights switched off. Neither are his doctors at Kennedy Krieger Institute, a Johns Hopkins partner that helps children with developmental disabilities. They don't know why the sight of a trash bag or the sound of a toilet flush upsets him. This sensory sensitivity is possibly one indication of his mutated SYNGAP1 gene, and he's one of a couple of hundred known cases.
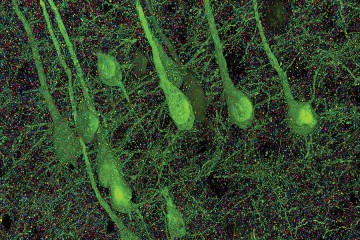
When Tyler was diagnosed at 16 months, it was hard for his mother when she realized how little science knew about the condition. They traveled from their Indiana home to meet doctors at Kennedy Krieger who were familiar with the mutation responsible for Tyler's sensory processing disorder, low muscle tone, and developmental delay. When a group of roughly 20 SYNGAP1 families met up in Baltimore last fall and visited Richard Huganir's lab at Johns Hopkins, McNamar was able to see tangible evidence for her son's difficulties: the SynGAP protein—a product of the SYNGAP1 gene—glowing green under a microscope. "I felt like I wasn't alone anymore," says McNamar, reassured that scientists were addressing the problem.
Huganir, the director of the Neuroscience Department at the School of Medicine and a newly appointed Bloomberg Distinguished Professor, first discovered the SYNGAP1 gene in mice in 1998 while studying the brain's synapses, the connections between neurons where thoughts become actions and memories form. One neuron releases signals, called neurotransmitters, that another neuron's receptor proteins detect. During learning, receptor proteins band together to form a scaffold that makes the neuron more responsive to neurotransmitters. Huganir's group was studying one such protein when they found SynGAP in the mix.
In 2009, his research was brought into focus through the work of Jacques Michaud, a neuroscience researcher at the University of Montreal. Michaud had identified three children with intellectual disability arising from mutated SYNGAP1 genes within either egg or sperm cells. Since Michaud's discovery, Huganir and others have concentrated their efforts on unraveling the complex nexus of SYNGAP1 mutations, determining the condition's associated traits, and identifying possible therapeutics. Abnormal synapses have been implicated in not only intellectual disability but also autism, schizophrenia, bipolar disorder, and Alzheimer's disease. Researchers believe that understanding the ramifications of one gene mutation, such as SYNGAP1, offers a window into the others.
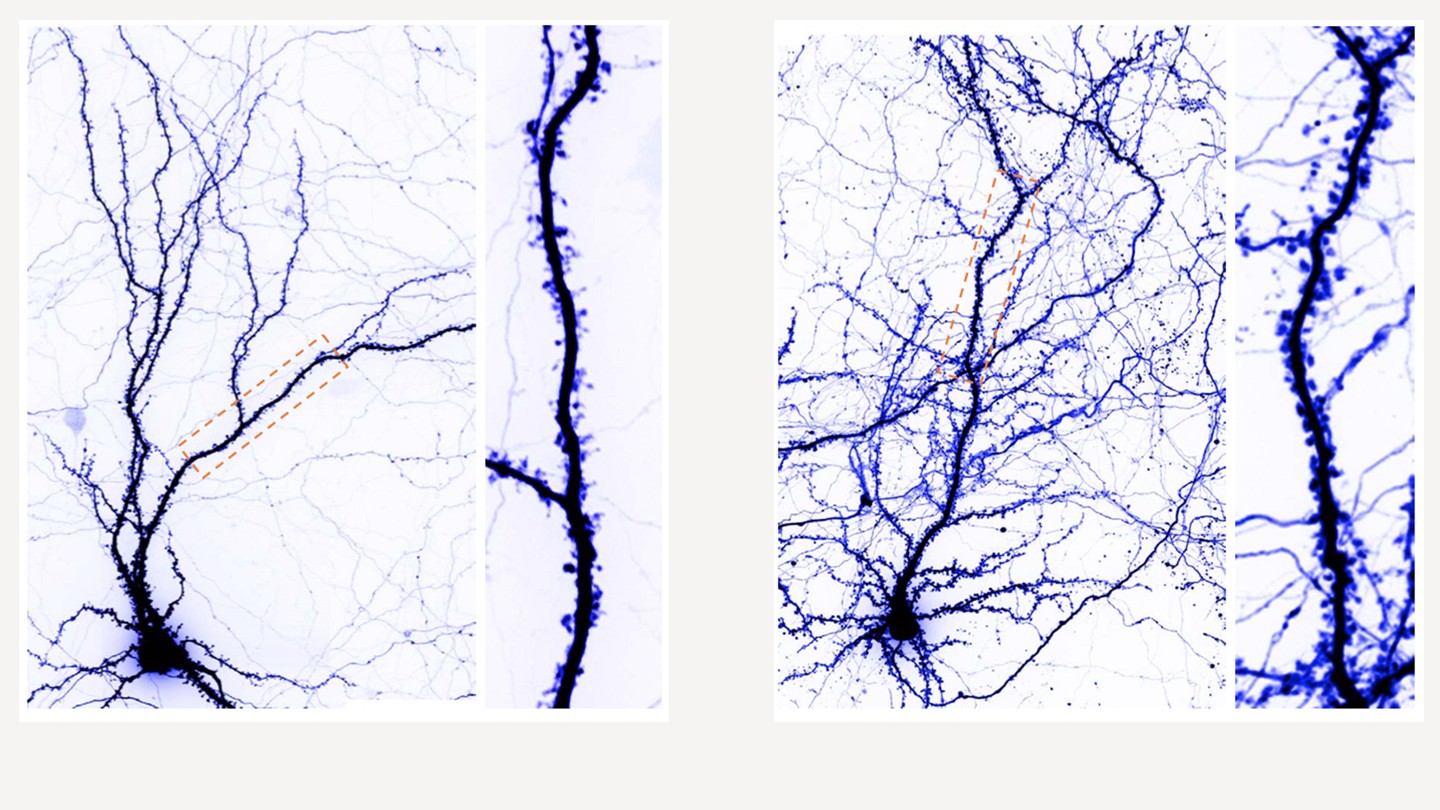
Image caption: Images above show normal neurons (left) and the enlarged dendritic spine of neurons with SYNGAP1 mutations
Image credit: Courtesy of Richard Huganir
While this diagnosis can be isolating, each new member of this tiny but growing patient community is key to elucidating SYNGAP1-related intellectual disability. Monica Weldon, another SYNGAP1 mom, founded Bridge the Gap, the research foundation responsible for the meet-up last November. Through the foundation, she and other parents have paved the way for collaboration involving funding agencies, researchers, and clinicians—including several at Johns Hopkins."I knew if I didn't try to do everything I could to help my son and help others like him or find others like him, I would regret it," Weldon says.
After the family meet-up, Kennedy Krieger established a multidisciplinary SYNGAP1 clinic where specialists diagnose children and help them with learning, walking, speaking, other everyday tasks, and behavioral issues. With the help of Bridge the Gap and scientists at Baylor College of Medicine in Texas, Kennedy Krieger is undertaking what's called a natural history study. This involves examining these children to note whether they have autism, epilepsy, and difficulty walking, talking, learning, or sleeping—facets of the condition that most patients face to varying extents. "The primary questions are how can we be better informed and provide optimal care to patients and how can we prepare ourselves for doing clinical trials when the time is right," says Constance Smith-Hicks, co-director of the SYNGAP1 clinic.
In one drug screening conducted at the Johns Hopkins medical campus, Huganir's team treated neurons growing in a dish with statins, the medication commonly used to lower cholesterol, to see how synapses changed. Like patients, these neurons had one normal SYNGAP1 gene and one abnormal copy. When one copy of the gene is mutated, lower levels of the protein are produced and that causes synapses to enlarge, damping their ability to form new connections in the brain. Huganir found that statins restore these abnormal synapses to their regular size. "Then, they can respond, they have the dynamic range to get bigger and smaller," Huganir says. It is this dynamism that helps shape a neuron's response to a stimulus.
The experiences of SYNGAP1 families are cluing researchers into the patterns of the mutation, helping take science "from the bench to the bedside and back," Smith-Hicks says.
As for the families, they have gained not only a support system but an understanding of the snail-paced nature of science and drug development. While researchers work, families cope as best they can. Whenever they're in a public restroom, Virginie McNamar says she must prepare Tyler for the imminent flushing of a public toilet—a warning to protect his ears.
Posted in Science+Technology
Tagged genetics, neuroscience, rick huganir