- Name
- Amy Mone
- amone@jhmi.edu
- Office phone
- 410-614-2915
The U.S. Food and Drug Administration on Tuesday approved a new drug for brain cancer that stems from a 2008 genetic discovery made by researchers at the Johns Hopkins Sidney Kimmel Cancer Center.

Image caption: Bert Vogelstein
This drug is for previously untreatable low grade IDH-mutant glioma, the most common malignant primary brain tumor in adults. This is the first major advance in decades for patients diagnosed with low grade gliomas, a malignancy of the brain's glial cells, which help control brain function, that occurs most commonly in younger adults. Low grade gliomas tend to be slower growing and are associated with longer survival than aggressive, high-grade brain cancers.
The drug, called vorasidenib, is a targeted cancer therapy that works by inhibiting the activity of a mutated gene called IDH, slowing the growth of the cancer. The gene was identified by Bert Vogelstein and team at the Johns Hopkins Kimmel Cancer Center's Ludwig Center in 2008 when, working with Duke University colleagues, they became the first to map the genetic blueprint for brain cancer. The blueprint was considered the most comprehensive genetic analysis for any tumor type, evaluating all known protein-encoding genes in brain cancer.
The researchers found that the IDH gene—which was never suspected to be involved in any tumor type—was frequently mutated in a subset of brain cancers.
"IDH is the poster child for cancer genome sequencing, and it illustrates the importance of basic research," says Vogelstein, professor of oncology and co-director of the Ludwig Center at Johns Hopkins. "The history of medicine shows that when a disease is understood, it eventually becomes manageable. It may not be immediately evident, but in time, as in this case, such discoveries result in better treatment for patients."
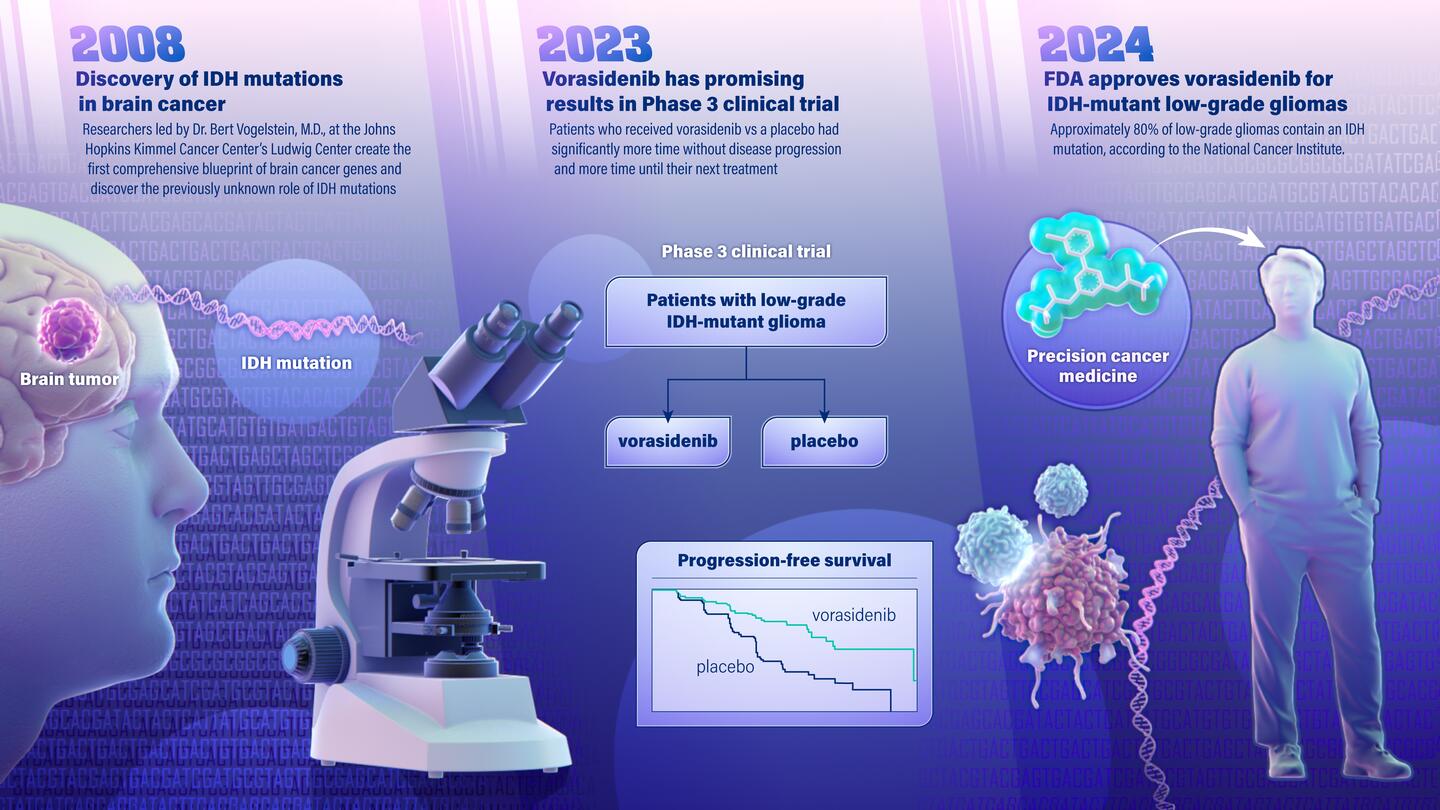
Image credit: Elizabeth Cook
In June 2023, findings from a multicenter, phase 3 clinical trial of vorasidenib in 331 patients with IDH-mutant low grade glioma published in the New England Journal of Medicine concluded:
- That patients with grade 2 IDH-mutant glioma who received the drug had significantly improved progression-free survival.
- That the therapy delayed the time to the next intervention compared to patients who received a placebo.
This phase 3 clinical trial was sponsored by the global pharmaceutical company Servier, which is bringing vorasidenib to market. The study showed that patients who received vorasidenib lived much longer without disease progression than participants who received a placebo.
"The discovery of the mutated IDH gene has become one of, if not the most important advancements in the entire field of neuro-oncology. Its use by the World Health Organization for accurate diagnosis and classification of malignant gliomas is groundbreaking and unparalleled," says longtime collaborator Darell Bigner, professor of neurosurgery at the Duke University School of Medicine and founding director of the Preston Robert Tisch Brain Tumor Center. "The drug vorasidenib is the first effective new drug developed in the last 30 years for treating malignant gliomas. The brain tumor centers at Duke and Johns Hopkins provide amazing connections to patients for this kind of research and hopefully they can reap the benefits of this diagnostic and therapeutic advancement."
In addition to this newly FDA-approved drug, the IDH gene discovery led to a new classification of gliomas, differentiating cancers with an IDH mutation that have overall better outcome and response to treatment from very aggressive gliomas without an IDH mutation, including glioblastoma, the most common primary brain cancer in adults. It has also paved the way for additional studies in other types of brain cancer.
Treatments include surgery to remove as much of the tumor as possible, followed by chemotherapy and radiation therapy to attack remaining cancer cells. In some patients, the use of the IDH inhibitor could delay the need for radiation and chemotherapy, which can damage healthy brain cells in the vicinity of the tumor and may render the tumor more aggressive over time.
"The possibility of delaying radiation therapy with this drug could be beneficial to select patients with slow growing IDH-mutant gliomas.," says Matthias Holdhoff, co-director of the Johns Hopkins Kimmel Cancer Center brain tumor program and a co-investigator on the 2023 clinical trial. "I believe we are looking at a new standard of care option for these types of tumors."
Added Christy Wyskiel, who oversees commercialization at Johns Hopkins: "The distance between early discovery and the marketplace is enormous and treacherous for new medicines. We celebrate the movement of assets through clinical trials, but most fail along the way to meet our national standards of safety and efficacy. The FDA's announcement about the approval of vorasidenib is a rare and beautiful moment when we can truly say we have been successful in translating academic discovery for the benefit of human health."
Tagged cancer, brain cancer, bert vogelstein, technology ventures









