- Name
- Lisa Ercolano
- lde@jhu.edu
Johns Hopkins University is joining forces with Yale University to establish a new center featuring groundbreaking technology for imaging, studying, and developing new treatments for tumors.
Supported by a National Cancer Institute grant, the Johns Hopkins Center for 3D Multiscale Cancer Imaging will be a hub for interdisciplinary research and collaboration between oncologists, engineers, pathologists, and computer scientists. The goal is to unlock the mysteries surrounding tumorigenesis (the formation of tumors) through the integration of cutting-edge molecular and cellular analysis.
"Our multidisciplinary team of clinicians, computational biologists, and bioengineers will develop computer algorithms combined with advanced molecular tools to identify and map in three dimensions the key types of cells responsible for the spread of cancer," said center co-founder Denis Wirtz, professor of chemical and biomolecular engineering, vice provost for research at Johns Hopkins, and a core researcher at JHU's Institute for NanoBioTechnology.
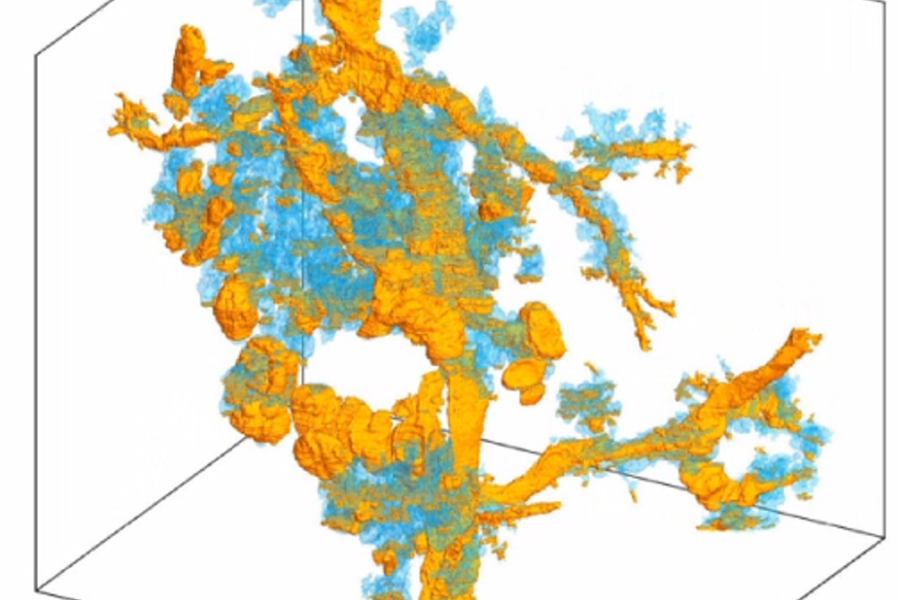
Researchers at the new center will use CODA, a cutting-edge computational research platform co-developed by PhD student Ashley Kiemen and Pei-Hsun Wu, an associate research professor in chemical and biomolecular engineering and the INBT, that has the ability to turn tissue slides into a 3D model—a process that creates hundreds of images of ultrathin tissue sections and aligns them with extremely high accuracy, allowing researchers and clinicians to manipulate and examine them in ways never before possible.
Scientists have used sectioning to study the tissue of plants, humans, and other organisms for many years. By using specialized tools to slice tissue at specific thickness, adhering the sections to a slide, and then reassembling the slides, researchers have been able to observe in 3D how a plant's stem or a human being's brain tissue is structured. The power of modern-day super computers and artificial intelligence will enable the new center's researchers to build and expand on this method to study tissue structures, cells, and their characteristics at the nano level.
"Our CODA system will allow us to produce 3D models by assembling sections of tissue samples so small we can see the anatomy of each cell and so large we can assess extremely large volumes of tumors," said Laura Wood, associate professor of pathology and oncology at Hopkins Medicine, director of gastrointestinal and liver pathology, associate researcher at the Institute for NanoBioTechnology and center co-founder. "We believe this will give us a revolutionary ability to see, for example, the precise location where cancer cells enter the bloodstream to initiate metastasis."
CODA breaks new ground with its ability to not only perform the slide assembly process in a matter of minutes, but also because it creates an algorithm that automatically assigns characteristics to every part of the tissue on each slide, such as counting individual cells in each tissue region. As a result, a comprehensive 3D model of large pieces of tissue is created, giving researchers an unprecedented view and understanding of human tissue—including tumors.
The center's team believes that the insights CODA provides on tumor micro-anatomy, composition, and vascularity could be transformative in terms of cancer diagnosis and treatment.
Initially, researchers will focus on pancreatic and breast cancer, two of the most common and deadliest types of cancer worldwide. As a pancreatic cancer researcher, Wood has experience working with patients living with cancer and undergoing treatment.
"These two cancer types have enormous clinical impact, and both are in dire need of the insights we can provide with our 3D multiscale imaging," said Wood. "It's my hope we can work towards the development of new diagnostic and therapeutic approaches to improve patients' lives and outcomes."
Wood says pancreatic cancer has the worst outcome of any common tumor type, often spreading throughout a patient's body and with few symptoms to alert a patient or doctor to its presence. In both pancreatic and breast cancer, once tumors reach the liver, most patients do not survive. A detailed 3D image of a tumor would give researchers the ability to see exactly where its cells are accessing the bloodstream, contributing to metastasis. Wirtz says removing tumors from a patient whose cancer has spread can sometimes be successful, but if cells or the part of the tumor responsible for metastasis remain in the body, that tumor can spread again.
"With CODA's capabilities, the team will be able to reconstruct the entire tumor and not only determine where the tumor is accessing the blood supply, but also what exactly happens the moment a tumor cell finds that blood vessel and enters it," said Wirtz.
Along with colleagues in pathology and engineering, Wood and Wirtz have been working together for years, laying the groundwork for the specialized 3D imaging methodology culminating in this major center grant from the National Cancer Institute. Wirtz credits advancements in artificial intelligence for their ability to develop CODA and use it to learn see tumors in a way that has never been possible before. But both he and Wood say it's the center's people—who include School of Medicine's Andrew J. Ewald, a professor of cell biology; Elana Fertig, an associate professor of oncology; and Ralph Hruban, professor of pathology; and physicist and data scientist Alex Szalay from the Krieger School of Arts and Sciences—that will ultimately make it a landmark institution.
The center is supported by a U54 center grant of the National Cancer Institute.