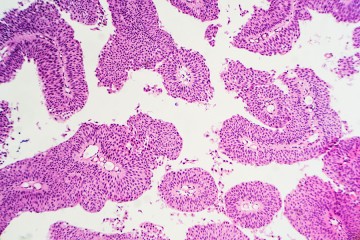
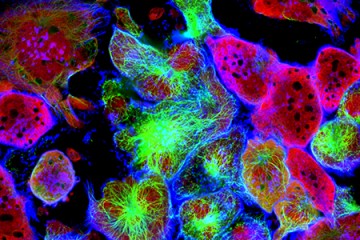
Scientists at the Johns Hopkins Kimmel Cancer Center have discovered a biochemical process that may give prostate cancer cells the ability to change their shape, squeeze into other organs, and take root in other parts of the body. Their findings, the scientists say, suggest new ways to intercept or reverse metastasis—the ability of cancers to spread.
For the study, the scientists mined public research data from five studies of men with prostate cancer, examining the genetics and chemistry of cancer samples collected in those studies. They found that a gene called "absent in melanoma 1," or AIM1, is deleted in approximately 20-30 percent of prostate cancers confined to the gland. In metastatic prostate cancers, the AIM1 gene is deleted about 40 percent of the time.
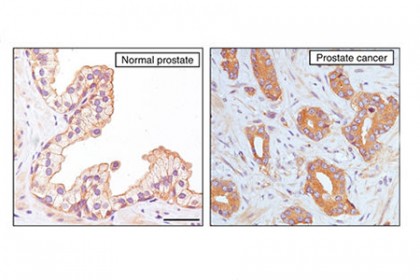
Image caption: AIM1 proteins are stained brown in normal prostate cells and are located along the outside border of each cell (left image). AIM1 proteins in primary prostate cancer cells diffuse throughout each cell (right image).
Image credit: Nature Communications
In addition, the scientists found that metastatic prostate cancer cells have far less of the AIM1 protein than do normal prostate cells or cells from men with cancer confined to the prostate, suggesting that reduction of AIM1 proteins is linked to tumor spread.
The findings are described online in the journal Nature Communications.
"Our experiments show that loss of AIM1 proteins gives prostate cancer cells the ability to change shape, migrate, and invade," says Michael Haffner, a pathology resident and former postdoctoral fellow at the Johns Hopkins Kimmel Cancer Center who is involved in the research. "These abilities could allow prostate cancer cells to spread to different tissues in an animal and, presumably, a person. It's not the whole story of what is going on in the spread of prostate cancer, but it appears to be a significant part of it in some cases."
The research team used dyes to track the movement of AIM1 proteins throughout different types of cells. They examined normal prostate cells, cells from prostate tissues with localized cancer, and cells from metastatic prostate cancers that had spread to the lymph nodes. In normal prostate cells, AIM1 was located along the outside border of each cell and paired up with a protein called beta-actin that helps form the cell's cytoskeleton, or scaffolding. However, in the cancerous cells, the protein spread away from the outer border of the cells and no longer paired up with beta-actin.
"It appears that when AIM1 protein levels drop, or when it's abnormally spread throughout the cell instead of confined to the outer border, the prostate cancer cells' scaffolding becomes more malleable and capable of invading other tissues," says Vasan Yegnasubramanian, associate professor at the Kimmel Cancer Center and a member of the research team. Yegnasubramanian says that with AIM1, the scaffold keeps normal cells in a rigid, orderly structure. Without AIM1, cells become more malleable, shape-shifting nomads that can migrate to other parts of the body.
This discovery raised questions about how these shape-shifting cancer cells—freed from their rigid cytoskeleton scaffolding—move in a tissue. The team enlisted Steven An, an expert in cellular mechanics and an associate professor at the Johns Hopkins Bloomberg School of Public Health, to take an up-close look at AIM1-lacking prostate cancer cells and use sophisticated and quantitative single-cell analyses to probe the material and physical properties of the living cell and its cytoskeleton.
They found that cells lacking AIM1 remodeled their scaffolding more than twice as much as cells that had normal levels of AIM1, and that they exert three to four times more force on their surroundings than cells with normal levels of the protein. These cells could also migrate to unoccupied spaces on a culture dish or invade through connective tissue-like materials at four times the rate of cells with normal levels of AIM1.
In mouse studies, the cells with depleted AIM1 spread to other tissues at levels 10 to 100 times higher than cells with normal levels of AIM1. However, the AIM1-lacking cells were not able to establish full colonies and tumors in those other tissues, suggesting that AIM1 depletion is not the whole story in the spread and growth of metastatic prostate cancer.
"AIM1 may help prostate cancer cells disseminate throughout the body, but something else may be helping them form full-blown metastatic tumors when they get there," says Yegnasubramanian.
Read more from Hopkins MedicinePosted in Health, Science+Technology
Tagged cancer, prostate cancer, kimmel cancer center, cancer metastasis