Johns Hopkins researchers report evidence that the natural ability of zebrafish to regenerate retinal tissue in their eyes can be accelerated by controlling the fishes' immune systems.
The new findings may one day advance efforts to combat degenerative eye disease damage in humans, the scientists say, because evolution likely conserved this mechanism of regenerative potential in other animals. A report on their experiments was published in April in Proceedings of The National Academy of Sciences.
"At the cellular level, zebrafish and human eyes are remarkably similar," said Jeffrey Mumm, associate professor of ophthalmology at the Johns Hopkins University School of Medicine.
Both human and zebrafish eyes contain Müller glia, an "inducible" stem cell type that gives zebrafish their remarkable regenerative abilities. The researchers say they found evidence that microglia, a cell type found in most vertebrae innate immune systems, affect the Müller glia's regenerative response and can be harnessed to accelerate the growth of new tissue in the retina.
For the study, researchers created a model of the human degenerative retinal disease retinitis pigmentosa in zebrafish by incorporating a gene for a specialized enzyme into the rod cells of the fish retina. The enzyme has the novel ability to convert a chemical (metronidazole) into a toxin, allowing researchers to selectively kill the cells expressing it.
After initiating photoreceptor loss in the fish retinas, the researchers monitored the immune system's response by tracking the activity of three types of fluorescently labeled immune cells in and around the eye: neutrophils, microglia, and peripheral macrophages. They were able to follow the activity of the immune cells using time-lapse 3-D microscopy imaging of fish retinas.
They found that neutrophils, the type of immune cells that are typically the first responders to tissue injury, were largely unresponsive to photoreceptor death. They also observed that the peripheral macrophages sensed the injury but were unable to penetrate the blood-retinal barrier to access the dying cells.
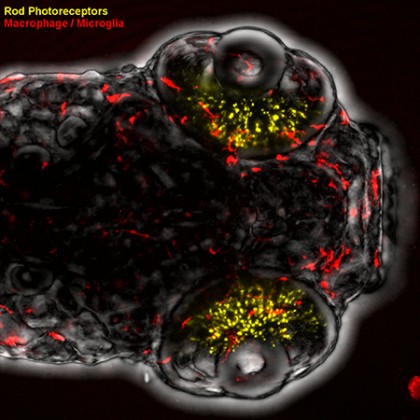
Image caption: Zebrafish larvae and fluorescently marked immune cells allow researchers to track immune system activity in a model of retinal degeneration.
Image credit: David White / Hopkins Medicine
Microglia were the only cells the researchers saw that were able to both respond to the injury and reach the injured cells.
"We could see the peripheral macrophages wanted to do something, but could not gain access," Mumm said. "Neutrophils didn't even detect that something had happened, but the microglia were at the right place at the right time."
Building on that evidence, the researchers conducted tests in zebrafish with the specialized enzyme incorporated into both rod cells and microglial cells, removing both cell types to ask what role microglia play during regeneration. They found that when microglia were also lost, Müller glia showed almost no regenerative activity after three days of recovery, compared with approximately 75 percent regeneration in the control population.
The researchers hope that by harnessing the ability to improve regeneration in zebrafish, they can better understand how to induce regeneration in human eyes, which share many of the same mechanisms for controlling regenerative potential. In the future, the researchers hope to improve their imaging techniques to build a more comprehensive understanding of how immune cells impact the regeneration process.
"Humans have an evolutionary block on our ability to regenerate certain tissues," Mumm said. "But humans still have the genetic machinery needed to regenerate retinal tissue, if we can activate and control it."
Funding for this research came from the National Eye Institute and the National Center for Advancing Translational Sciences.
Note: The value of Luminomics' products could be impacted by the outcome of this study. Mumm is a founder of Luminomics, and his spouse, Meera Saxena, owns shares of Luminomics' stock. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Read more from Hopkins MedicinePosted in Health, Science+Technology
Tagged ophthalmology








