Biomedical engineers at Johns Hopkins report they have worked out a noninvasive way to release and deliver concentrated amounts of a drug to the brain of rats in a temporary, localized manner using ultrasound. The method "cages" a drug inside tiny, biodegradable "nanoparticles," then activates its release through precisely targeted sound waves, such as those used to create images of internal organs.
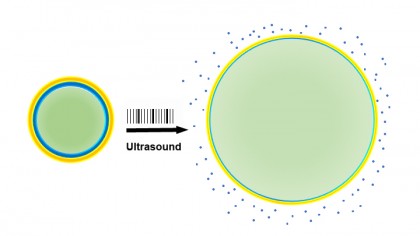
Image caption: When drug-laden nanoparticles (left) absorb energy from ultrasound waves, their liquid center (green) turns to gas and expands the particles (right), loosening their exterior and releasing the drug (blue)
Image credit: Raag Airan
Because most psychoactive drugs could be delivered this way, as well as many other types of drugs, the researchers say their method has the potential to advance many therapies and research studies inside and outside the brain.
The researchers say that their method should minimize a drug's side effects because the drug's release is concentrated in a small area of the body, so the total amount of drug administered can be much lower. And because the individual components of the technology—including the use of the specific biomaterials, ultrasound, and FDA-approved drugs—have already been tested in people and found to be safe, the researchers believe their method could be brought into clinical use more quickly than usual. They hope to start the regulatory approval process within the next year or two.
"If further testing of our combination method works in humans, it will not only give us a way to direct medications to specific areas of the brain, but will also let us learn a lot more about the function of each brain area," said Jordan Green, associate professor of biomedical engineering, who is also a member of JHU's Kimmel Cancer Center and Institute for NanoBioTechnology.
Details of the research were published Monday in the journal Nano Letters.
In their experiments, Green's group designed nanoparticles with an outer expandable "cage" made of a biodegradable plastic. The center of the cage was filled with the liquid perfluoropentane. When the sound waves of ultrasound—delivered noninvasively across the rats' scalp and skull—strike perfluoropentane in the center of the nanoparticles, the liquid transforms to a gas, expanding the surrounding cage and letting the drug escape.
The new research, Green says, was designed to further advance means of getting drugs safely to the brain, a delicate and challenging organ to treat. To protect itself from infectious agents—and from swelling that can be caused by the immune system, for example—the brain is surrounded by a molecular fence, called the blood-brain barrier, which lines the surface of every blood vessel feeding the brain. Only very small drug molecules that dissolve in oil can get through the fence, along with gases. Because of this, most drugs developed for treating brain disorders fit those criteria but are dispersed to all parts of the brain—and the rest of the body, where they may be unneeded and unwanted.
"When working with a patient who has post-traumatic stress disorder, for example, it would be nice to quiet down the overactive part of the brain—for instance, the amygdala—during talk therapy sessions," says Raag Airan, assistant professor of radiology at Stanford University Medical Center and co-author of the paper. "Current technologies can at best quiet down half of the brain at a time, so they are too nonspecific to be useful in this setting."
Read more from Hopkins Medicine