Johns Hopkins researchers have begun to connect the dots between a schizophrenia-linked genetic variation and its effect on the developing brain. As they reported earlier this month in the journal Cell Stem Cell, their experiments show that the loss of a particular gene alters the structure of developing brain cells, which in turn disrupts the orderly layers those cells would normally form.
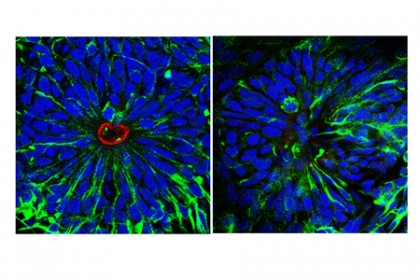
Image caption: Human neural stem cells (left) form rosettes as they grow into different cell types, with ringlike patterns in the center. A neural rosette with a 15q11.2 microdeletion (right), a risk factor for schizophrenia, appears disorganized and lacks the ringlike structure, suggesting that this risk factor acts early in the neurodevelopmental process.
Image credit: Ki-Jun Yoon/Johns Hopkins Medicine
"This is an important step toward understanding what physically happens in the developing brain that puts people at risk of schizophrenia," says Guo-li Ming, a professor of neurology and neuroscience in the Johns Hopkins University School of Medicine's Institute for Cell Engineering.
While no single genetic mutation is known to cause schizophrenia, studies have identified variations that are more common in people with the condition than in the general population. One of these is a missing piece from an area of the genome labeled 15q11.2. For their study, Ming's research group—along with that of her husband and collaborator, neurology and neuroscience professor Hongjun Song—used skin cells from people with schizophrenia who were missing part of 15q11.2 on one of their chromosomes. (Because everyone carries two copies of their genome, the patients each had an intact copy of 15q11.2 as well.)
The researchers grew the human skin cells in a dish and coaxed them to become induced pluripotent stem cells, and then to form neural progenitor cells, a kind of stem cell found in the developing brain.
"Normally, neural progenitors will form orderly rings when grown in a dish, but those with the deletion didn't," Ming says.
To find out which of the four known genes in the missing piece of the genome were responsible for the change, the researchers engineered groups of progenitors that each produced less protein than normal from one of the suspect genes. The crucial ingredient in ring formation turned out to be a gene called CYFIP1, which plays a role in building the skeleton that gives shape to each cell. Many people with a CYFIP1 deletion do not get schizophrenia, but the team found that a variation in the signaling gene ACTR2/Arp2, combined with the CYFIP1 deletion, increased the risk of schizophrenia more than either genetic change by itself.
In adding to science's understanding of schizophrenia, the study also shows how other mental illnesses might be similarly investigated, the researchers say.
"Using induced pluripotent stem cells from people with schizophrenia allowed us to see how their genes affected brain development," Song says. "Next, we'd like to investigate what effects remain in the mature brain."
Read more from Hopkins MedicinePosted in Health
Tagged brain science, mental health








