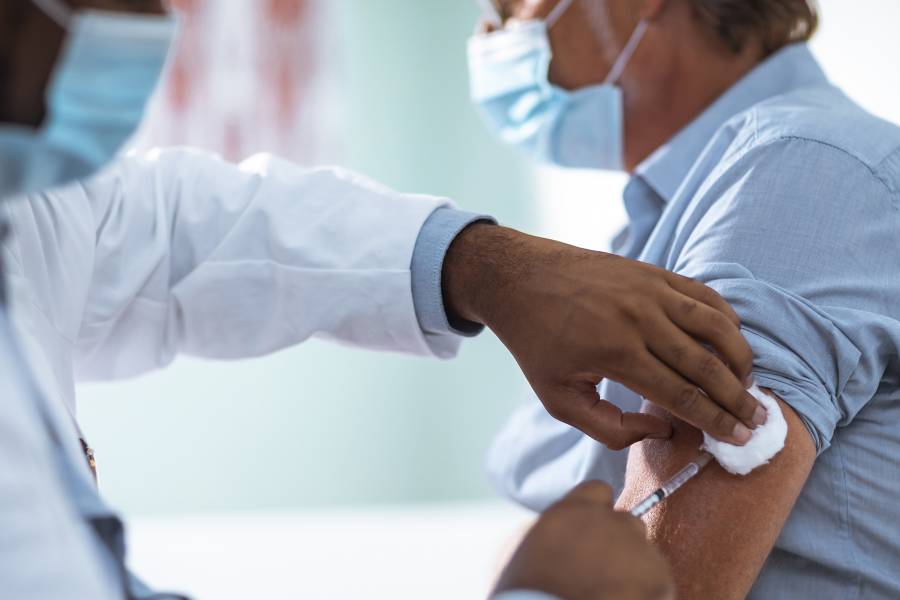
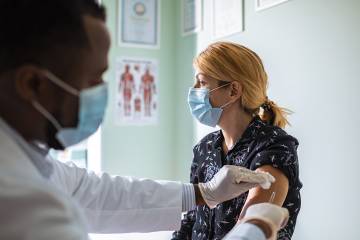
At last, an effective COVID-19 vaccine seems within reach. Earlier this week, Pfizer announced promising results from phase III clinical trials of the vaccine candidate it's developing with German pharmaceutical firm BioNTech SE, with preliminary analysis indicating efficacy of more than 90%.
Though many steps remain before the vaccine is widely available—including completion of the trial, peer review of full data, and approval by the FDA and other authorities—the companies intend to produce 50 million doses in 2020 and another 1.3 billion next year. Scientists have generally greeted the news with optimism while also cautioning the public that the vaccine won't bring an immediate or wholesale cure to the global pandemic.
To assess the implications of this milestone, the Hub sought insight from infectious disease expert David Dowdy and William Moss, executive director of the International Vaccine Access Center. Both are affiliated with the Johns Hopkins Bloomberg School of Public Health.
What does 90% vaccine efficacy mean?
Dowdy: It means that, if you took a group of people who had not previously had COVID-19, give half of them the vaccine and half a placebo injection, the group getting the vaccine would end up having 90% fewer cases of COVID-19 than the placebo group. In the case of the Pfizer vaccine, there were 94 cases of COVID-19 among people who participated in this interim analysis. Assuming roughly equal numbers got the vaccine and the placebo, this means probably about 86 of these cases happened in people who got the placebo, versus only about eight among those who got the vaccine.
Is that better than expected?
Dowdy: If these results hold up in the final analysis, then yes. I think most scientists were hoping for an initial vaccine that would be at least 50-60% effective (similar to most seasonal flu vaccines), so 90% would far exceed those expectations. It also suggests that, if one vaccine can reach this level of effectiveness, other COVID-19 vaccines in the pipeline might be equally effective.
Is there cause to temper the enthusiasm?
Dowdy: There's no reason not to trust these early numbers—they were released by an independent data monitoring committee, and putting out misleading figures would not be in Pfizer's interest. But early results are often more optimistic than final numbers. We need to wait until the final results can be reviewed by the scientific community as a whole.
Moss: The enthusiasm is warranted in that we're likely to have a vaccine that protects against COVID-19 in most vaccinated individuals, at least in the short term if not longer. However, much remains unknown. These are preliminary results shared through a press conference. Pfizer's trial is still ongoing and the full data are not yet public.
What important details do we still need to find out?
Moss: We need to know the vaccine efficacy in those who are most vulnerable to severe COVID-19, including older adults and people with underlying medical conditions. Also, so far we only have an idea of short-term effects. A longer follow-up study of participants will be necessary to see how long any vaccine-based immunity lasts, and to watch for any unexpected and possibly serious side effects that may only become apparent months after vaccination.
How long will it take for this vaccine to become available to the public?
Dowdy: We still have a long way to go. The next steps will be to finish the trial, analyze the data, and report those data to the FDA and other authorities, before moving on to wide-scale production and distribution. This is not going to be an easy vaccine to distribute—it requires storage at very cold temperatures, for example—and manufacturing billions of vaccine doses takes time and careful precision. It's going to be at least several months before any members of the general public get this or any other COVID-19 vaccine.
Also see
What does the Pfizer news mean for other COVID-19 vaccines in development?
Dowdy: We can expect other vaccine manufacturers to continue their testing. The best way to produce vaccines quickly is to test many vaccines simultaneously—which is exactly what's happening. If this first vaccine can achieve high levels of effectiveness, there's reason to believe that other vaccines might be able to do the same, and the ideal end result would be having more vaccines available to more people more quickly. Other vaccines might also have other advantages over this first vaccine—they might be effective in a single dose, for example, or have fewer cold storage requirements.
Moss: Ten other vaccine candidates are also in phase III clinical trials now, with many more in the pipeline. All of the vaccine candidates aim to induce immune responses to the spike protein of SARS-CoV-2, the protein on the surface of the virus that allows it to attach to our cells. What the Pfizer vaccine study shows is that this is very likely to be a winning strategy.
What may complicate other ongoing vaccine trials is whether they can continue using a placebo vaccine for comparison once a highly effective vaccine is approved. That's a complex ethical issue that will need to be resolved.
Are there any misunderstandings that you think need to be cleared up?
Dowdy: The public should understand that in general, vaccines are held to a very high standard of safety, since they're given to people who are otherwise healthy. So although we can never rule out very rare side effects, we can rest assured that if this vaccine is approved by the FDA, it's safe enough for you to take.
Moss: It would be a mistake to assume we'll be able to go right back to our pre-pandemic lifestyles when a vaccine is approved. That's not the case. Right now, rates of the virus are the highest they've ever been in many parts of the U.S. and Europe. It's important to continue with steps to prevent transmission—wearing masks, maintaining physical distance, avoiding large gatherings, and regularly washing hands—until and even after a vaccine becomes available. Then we'll need to wait to deliver it to a large proportion of the public and ensure that it offers long-term protection from COVID-19.
Posted in Health, Science+Technology
Tagged vaccines, pharmaceuticals, covid-19